If you’d like to know more about the essential role that light plays in photography, then you’ll love our course, A Photographer’s Guide to Light. In this lesson, you’ll learn some of the basics of light.
What Is Light?
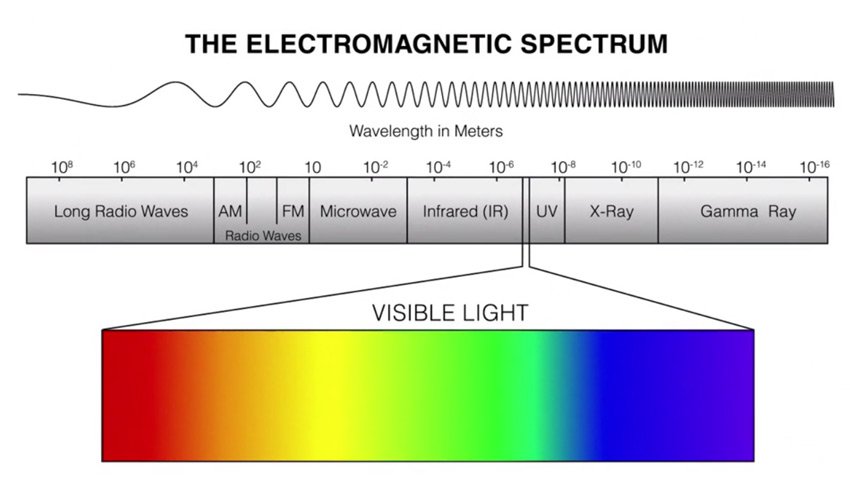
When we use the word light, most of the time we’re referring to visible light, light that is visible to the human eye. The kind of light that makes our sense of vision possible. Visible light is only a portion of the electromagnetic radiation spectrum, however.



The larger spectrum of electromagnetic radiation includes: radio, microwave, infrared, the visible region that we perceive as light, ultraviolet, X-Rays and Gamma Rays.
Electromagnetic radiation, including the visible spectrum, behaves like a wave and like a particle. Light waves that encounter an obstacle can interfere with each other, which is called refraction.
In photography you may be familiar with this concept. If you close down your aperture too far, the light starts to create an interference pattern on your image sensor and your image will look softer. This is diffraction at work.
Light also acts as a particle. In the late 19th and early 20th century, experiments with light and metals showed that light could cause electrons to be emitted, or ejected from metals. This meant that light acted like a particle, and these particles were called photons.
This idea of light behaving like a wave and light behaving like a particle is all very complex stuff. The particle nature of light was first explained by Albert Einstein, but you don’t need to understand it on his level! It is important though, to understand at least at basic, how light acts as a particle and a wave, because it will help explain how light interacts with matter, like the things you want to photograph.
An Example
Sometimes it’s easier to think about light interacting with matter as a particle, and sometimes it’s easier to think about light interacting with matter as a wave. This isn’t some kind of scientific shortcut, it’s more about using the explanation that makes the most sense to those of us who haven’t spent our lives studying physics.



When a wave passes through an opening or a slit, the wave radiates outward with a spherical pattern. This apparent change in direction is called diffraction. If you do this with light, it creates a band of light on the wall behind the opening that’s larger than the slit.



With two slits, you would expect two bands of light on the other side, but actually what happens is something much different. The light coming through the slit creates an interference pattern on the wall. This is called the double slit experiment and in the early 1800s this experiment helped to solidify the acceptance of the wave theory of light.
Real Life Example of an Interference Pattern



In this experiment a green laser is pointed at two very narrow slits created by three pieces of graphite taped close together, they’re almost touching.



When the graphite is pushed front of the laser that same interference pattern is created.
Photoelectric Effect



Here, an aluminium can is connected to a steel wire, that’s connected to strips of aluminium foil. To charge up the metal with electrons, you can rub a plastic bag onto a PVC pipe, then if the pipe is touched to the strips of aluminium, the electrons will be transferred to the them and they’ll repel each other.



They do this because the can and strip apparatus now have a net negative charge and like-charges repel each other.
If a UV light is pointed at the can, the foil strips will relax.



That’s due to the UV light causing the electrons to be ejected from the can, neutralising the charge. If this was repeated with a green laser, nothing would happen, because green light doesn’t have the same energy as UV light no matter how bright it is. A very bright spotlight wouldn’t work either, because the amount of energy in light is related to its frequency, not the quantity.
UV light has a higher energy compared to visible light so it will cause the electrons to be ejected from the can in this experiment. Lower energy — and thus lower frequency — light, won’t have any effect at all.
The Science-y Bit
Einstein explained that electromagnetic radiation is made up of photons. Photons are tiny bits of energy that travel through a vacuum at the speed of light. Photons have no mass and carry a certain quantifiable level of energy, which is also related to the frequency of the electromagnetic wave.
The electromagnetic field around the photon fluctuates from positive to negative and back to positive as the photons move through space. This can be thought of as a self-propagating transverse oscillating wave.
Self-propagating refers to the fact that a changing electric field generates a changing magnetic field and the changing magnetic field generates a changing electric field. The result is that it creates an infinite loop that self-propagates through space.



A transverse wave, is a moving wave that consists of oscillations occurring perpendicular to the direction of the wave. If a wave is moving along the X-axis, oscillations have to be in the Y-axis or the Z-axis. Electromagnetic waves have two components: An electric field and a magnetic field, that are oriented 90 degrees from each other and they are always in phase.



In this example, the electric field is in the Y-axis and the magnetic field is in the Z-axis. The wave is traveling in the X-axis.
Looking at more examples of how these waves work, it gets confusing to look at both fields at the same time, and it’s often good enough to just talk about the direction and orientation of the electric field.



This wave has a lot of similarities to other waves that you might be familiar with, like water waves. It has crests and troughs and the distance between them is the wavelength.
Frequency is the number of complete wave cycles or wavelengths that pass a point in space In one second. This measurement is called Hertz, after Heinrich Rudolf Hertz a German physicist who first conclusively proved the existence of electromagnetic waves. These waves were theorised by James Clerk Maxwell’s electromagnetic theory of light.



Unlike water waves, the faster an electromagnetic wave oscillates, or the higher the frequency, the more energy the wave has. In the visible spectrum, we see these differences in frequency as colour.
Red, has the longest wavelength and therefore, the lowest energy of visible light. Violet, is the highest frequency, and has the highest energy of visible light. Just above violet is ultraviolet light.
Ultraviolet light, has even more energy than visible light, and this is where electromagnetic waves start to become harmful to humans.



As you move up the scale, you get into X-Rays and gamma rays, which are even more harmful. As you move down the scale, just past visible light, you get to infrared, then microwaves, and finally long radio waves.
Infrared, is often associated with heat. This is because infrared energy causes molecules to vibrate faster, and we feel this as heat. In fact, everything in the universe is emitting infrared light. Unlike other forms of energy, electromagnetic radiation doesn’t need a medium to travel through. Electromagnetic radiation can travel through a vacuum.
More Light in Photography Guides



Photographer’s Guide to Light: Noticing Direct Reflections



A Photographer’s Guide to Contrast — How to See Light and Shadow



A Photographer’s Guide to Transmission — Understanding How Light Filters Through



Playing With the Qualities of Light in Studio Portrait Photography
About the Authors
David Bode created the video course that includes this lesson. Dave is an expert on video and audio production, and he lives in the upstate NY area. He works as a camera operator, editor, inventor, motion graphics designer, recording engineer, and studio musician.
Marie Gardiner wrote the text version of this lesson and it was edited and published by Jackson Couse. Jackson is a photographer and the editor of the Photo & Video section of Envato Tuts+.